Abstract

Introduction
Diffuse large B cell lymphoma (DLBCL) is a heterogeneous group of aggressive B cell lymphomas, considering their biologic, pathological and clinical backgrounds. However, treatment of DLBCL is relatively homogeneous and standard, mainly based in the R-CHOP regimen. Several prognostic scores have been proposed for categorizing the risk and finding those with worse results with standard treatment, suitable to be treated with new schemes or drugs. The most important and widely used is the International Prognostic Index (IPI) proposed in 1993 and lately validated in the rituximab era (R-IPI). However, being a good prognostic score lacks the ability to identify a very high risk prognostic subset in the rituximab era. Trying to improve this situation several attempts have been made including NCCN-IPI or GELTAMO-IPI. In 1992, the MD Anderson Cancer Centre (MDACC) reported a prognostic score considering exclusively variables related to the tumor: the Tumor Score (TS). Two of them already present in IPI: high LDH and Ann Arbor stage III-IV but three different: high beta-2-microglobulin (B2M), bulky mass and presence of B symptoms. This index has not been studied in the rituximab era. Our aim is to validate the TS in the rituximab era and analyze its current potential role.
METHODS
From the nation-wide database of DLBCL of the Grupo Español de Linfoma y Trasplante de Médula Ósea (GELTAMO), we included for the validation those patients homogenously treated with R-CHOP and with all five TS variables available (n=1294). Patients had to be ≥ 18 years-old and a minimum of 1 year of follow-up; all histological subtypes of DLBCL and primary extranodal cases were acceptable, with the only exclusion of primary testicular or CNS sites. Failure-free survival (FFS) (including disease progression, no response to treatment or death events) and overall survival (OS) were analyzed with the Kaplan-Meier method and compared with the log-rank test. Cox Regression models were used for univariate and multivariate analysis. Comparisons between scores were performed with concordance probability estimates (CPEs).
RESULTS
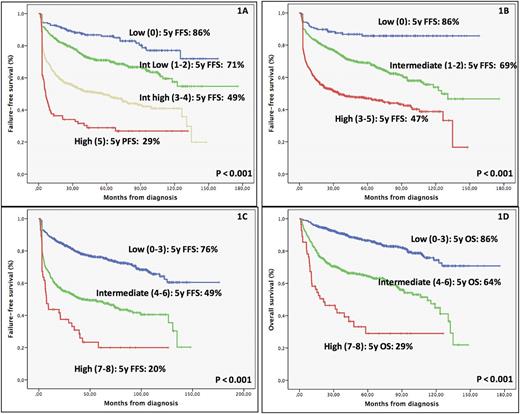
Median follow-up was 60 months (12-176). 5y-FFS and OS of the series were 62% and 74%, respectively. All the variables of the original TS retained an independent prognostic role in our series. The TS in the rituximab era (R-TS) remains predictive and clearly identifies four different risk groups (Figure 1A), finding a particularly high risk subset with a worse outcome (5y-FFS of 29%). Comparison between TS and the other indexes (IPI, NCCN-IPI or GELTAMO-IPI) showed similar CPEs for FFS in our series: 0.64 vs 0.64, 0.64 and 0.65, respectively (Figure 1B). However, TS has a better discrimination of the higher risk subgroup than IPI (5y-FFS of 47%) and NCCN-IPI (5y-FFS of 39%), and the same that GELTAMO-IPI but with a better consistency between points of the score. As R-IPI and R-TS have complementary information, another option was combining R-IPI and R-TS: patients within high risk by R-IPI (5y-FFS of 47%) when tested with R-TS can be further subdivided in three risk categories (intermediate low, intermediate high and high with 5y-FFS of 75%, 61% and 37%, respectively). To further improve the ability of finding a very high risk subset with the variables included in TS, we tested categorizing B2M normalized (normal, >1 and >3), LDH normalized (normal, >1 and >3) and AA stage (I, II and III-IV). With these changes the new enhanced TS could identify a higher risk group with a 5y-FFS of 20% and a median FFS of 7 months (Figure 1C and 1D).
CONCLUSIONS
1) All variables included in the original MD. Anderson TS retain an independent prognostic role in the rituximab era, including B symptoms, B2M and bulky mass; 2) TS remains predictive of FFS and OS in the rituximab era with a similar CPE in discrimination when compared to previously reported prognostic scores; 3) TS and GELTAMO-IPI showed a better identification of patients with high risk prognosis compared to IPI or NCCN-IPI; 4) R-IPI and R-TS may be combined in order to easily improve risk classification of DLBCL patients; 5) Further categorization of LDH, B2M and AA stage increased the ability of TS to identify high risk subsets of DLBCL; 6) TS could be a backbone for introducing other new molecular or biologic tumor related prognostic factors.
Figure 1. FFS using R-TS (1A), R-IPI (1B) and enhanced TS (1C). OS using enhanced TS (1D).
Gutierrez: SERVIER: Speakers Bureau; GILEAD: Honoraria; TAKEDA: Speakers Bureau; PFIZER: Consultancy; JANSSEN: Consultancy, Research Funding, Speakers Bureau; ROCHE: Research Funding, Speakers Bureau. Diaz-Lopez: TFS: Employment. Lopez-Guillermo: Roche: Consultancy, Other: Research grant; Celgene: Consultancy; Gilead: Consultancy; Janssen: Consultancy; Novartis: Consultancy. Martín: Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Janssen: Honoraria; Gilead: Consultancy. Salar: Roche: Speakers Bureau; Janssen: Speakers Bureau; Servier: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal